Sponsored by Endo Aesthetics
Endo Aesthetics is embarking on a mission devoted to pushing the boundaries of aesthetic artistry. Driven by world-class research and development, the company is advancing solutions to address unmet needs beginning with the launch of QwoTM (collagenase clostridium histolyticum-aaes), the first and only FDA-approved injectable for the treatment of moderate to severe cellulite in the buttocks of adult women.1
|
Steven Dayan, MD |
Cellulite is a localized alteration in the contour of the skin that has been reported in approximately 90% of adult women of all races and ethnicities.2,3 While cellulite is known to be a multifactorial condition, a primary contributing factor is the fibrous connective tissue, called the “fibrous septae,” which connects the skin perpendicularly to the fascia below.4,5 These fibrous septae tether the skin, drawing it downward and leading to a mattress-like appearance, commonly referred to as “dimpling.”6,7
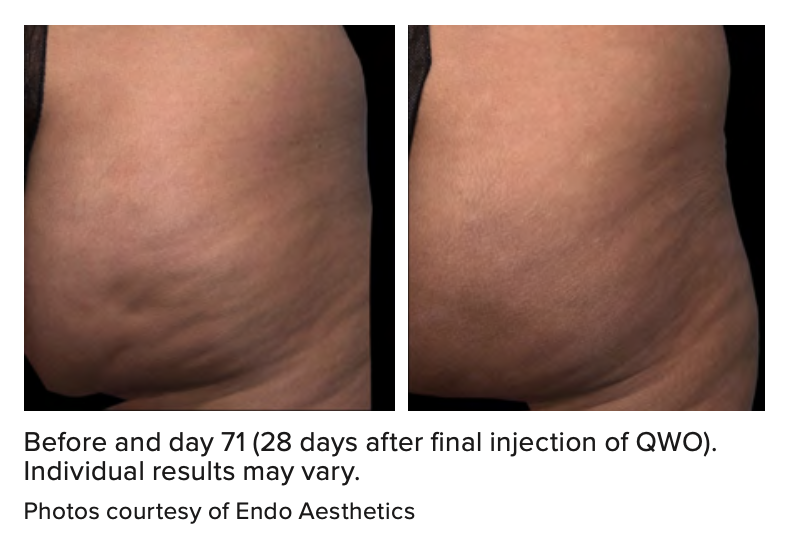
When QWO is injected into the treatment area, Enzymatic Subcision and RemodelingTM (ESRTM) begins. There are three components of ESR: enzymatic lysing of mature, collagen-rich septae, stimulation of neocollagenesis and fat lobule reorganization.8 ESR is believed to initiate as the enzyme works on the collagen in the fibrous septae to release the cellulite dimple.8 With the fibrous septae detached, the fat lobules begin to reorganize and spread out more evenly. This enzymatic activity induces or stimulates neocollagenesis, the building of new collagen.8 From within, this collagen support network rebuilds itself in the extracellular matrix with thinner, smaller septae.8 These subdermal changes reduce the tension and help thicken the dermis, yielding a smoother appearance.1,8
“I think there is currently a significant unmet need for an effective and non-invasive treatment option for cellulite, so I am excited that QWO will soon be available. This FDA-approved, injectable treatment will be a welcomed addition for those of us in medical aesthetics,” said Steven Dayan, MD, a QWO clinical investigator based in Chicago, Ill.
In clinical trials, the most commonly reported adverse reactions in patients treated with QWO, with an incidence of ≥ 10%, were at the injection site: bruising, pain, nodule and pruritus. Please see the QWO advertisement on page 14 in this publication for Important Safety Information.
Editor’s Note: QWO is expected to be available throughout the U.S. at aesthetic healthcare practitioners’ offices in spring 2021. Physicians and consumers are encouraged to visit www. QWO.com and sign up for updates on product availability.
References:
1. QWOTM package insert. Malvern, PA: Endo Aesthetics LLC
2. Hexsel DM, et al. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35(10):1471-7.
3. Khan MH, et al. Treatment of cellulite: Part I. Pathophysiology. J Am Acad Dermatol. 2010;62:361-70.
4. Zhang YZ, et al. Appl Environ Microbiol. 2015;81(18):6098-6107.
5. Rossi AM, Katz BE. Dermatol Clin. 2014;32(1):51-59.
6. Edkins TJ, et al. Clin Vaccine Immunol. 2012;19(4):562-569.
7. Kaplan FT. Drugs Today (Barc). 2011;47(9):653-667.
8. Endo Pharmaceuticals data on file 2020.

